Please note you need to be a West Moreton Health employee to submit projects within this category.
Please refer to the WMH research intranet page for more information and required forms.
Human Research Ethics Committee approval
Human Research Ethics Committee (HREC) approval is the first step in the research process, required for all human research conducted within West Moreton Health (WMH).
Please note WMH HREC is only able to review single site research studies (i.e. studies conducted within WMH).
Where the study is multi-site (i.e. includes sites outside of WMH), the application must be reviewed by an HREC that is certified for multi-centre review and participates in the National Health and Medical Research Council (NHMRC) National Mutual Acceptance (NMA) Scheme.
All research ethics applications (except for requests for exemption of HREC review) should be made using the Human Research Ethics Application (HREA) available via Ethical Review Manager (ERM).
Contact
Postal address
West Moreton Hospital and Health Service (HREC)
The Park - Centre for Mental Health
Locked Bag 500
Archerfield QLD 4108
Phone: 3413 7475
Email: WM_EthicsandGov@health.qld.gov.au
Ethics application process
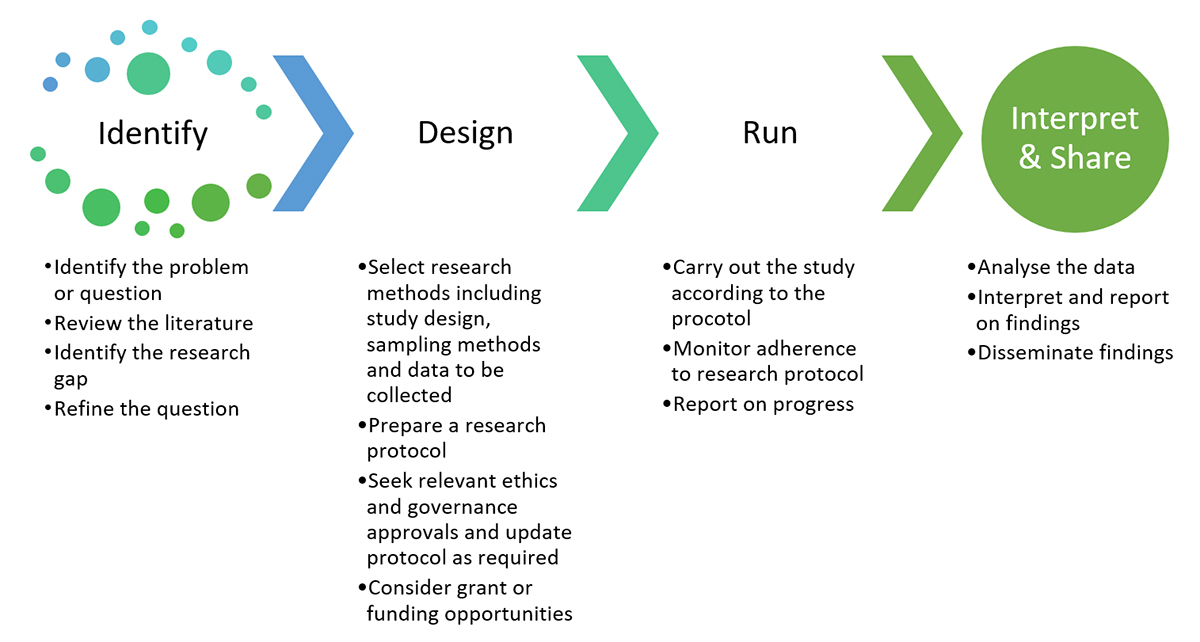
The steps outlined below are a guideline for the ethics application process.
1. Research protocol
A project protocol is an essential element of all applications and should be developed early in the research process. The research study protocol template may be useful in helping you develop your research protocol.
2. Participant information and consent forms
The NHMRC provides researchers with Participant information and consent form (PICF) templates. It is not compulsory to use these templates however they are useful tools and accepted by both HRECs located within Metro North. Standardised PICF templates can be obtained from the NHMRC website.
Be sure to incorporate Queensland Health branding into your PICF.
3. Ethics application
There are two types of application forms submitted to an HREC:
- Human Research Ethics Application (HREA), via ERM, for low and negligible risk (LNR) and greater than low risk research
- LNR research projects are reviewed out session by the HREC Chairperson and can be submitted at any time
- Greater than low risk research is reviewed at a meeting of the HREC and must be submitted by 12 noon on the closing date for the relevant meeting
- Request for exemption from ethics review, via ERM, for non-research activities (e.g. quality improvement, clinical audit, case study) that are intended to be published external to WMH
- Reviewed out of session by the HREC Chairperson and can be submitted at any time
4. Submission
Ensure all supporting documentation is uploaded to the ethics application form and submit to the HREC via ERM.
Applications to the WMHHS HREC must be submitted by 12 pm on the closing date.
5. Research governance/site-authorisation
After obtaining ethics approval from the Human Research Ethics Committee (HREC) applicable to your research, you must obtain site authorisation by submitting a site specific assessment (SSA) for research governance authorisation at all facilities you intend to conduct research.
6. Post approval/authorisation
Information on post approval/authorisation submissions can be found in the relevant section further down the page. In short, once the project has been authorised to commence, researchers need to:
- conduct the research project in accordance with the approved protocol
- If changes are required, submit an amendment to the approving HREC via ERM. Once approved, send a copy of the approval and documentation to the Principal Investigator at each site for submission to their local research governance office/r
- comply with ongoing monitoring and reporting requirements as outlined in the HREC approval and research governance authorisation letters
- Ongoing approval is contingent on the receipt of an annual progress report by 30 April each year
- implementation and dissemination of findings at project completion.
Research governance authorisation
It is a requirement of public health facilities within Queensland Health that research governance authorisation is granted before a research project can commence at a site. The purpose of the research governance review is to determine the suitability of a research study to a site and involves assessing the legal, financial, regulatory, and contractual matters related to the study.
The research governance is submitted using the Site-Specific Assessment (SSA) application via ERM. All SSA applications for West Moreton Health are reviewed by the Research Ethics and Governance Officers (REGOs), who then make recommendations to the appropriate delegates.
An SSA authorisation letter is required before a research study can commence at the site.
The Research Ethics and Governance Office can assist researchers with:
- SSA forms and the application processes for site authorisation, including relevant site contacts such as Heads of Department
- obtaining finance review and sign off from the relevant Finance Business Partner and Director of Financial Performance
- negotiation of research contracts, including obtaining West Moreton Health legal review where required
- guidance regarding legislation, policies and standards relating to the conduct of research, including access to confidential health information under the Public Health Act 2005
- education and training for new and experienced researchers to enhance the quality and conduct of research in accordance with good practice guidelines
- Monitoring of research, including responding to concerns about research conduct.
Obtain approval from an HREC
Whilst approval from a Human Research Ethics Committee (HREC) is required before a research study can be authorised, the ethics and governance processes can be conducted in parallel.
Site-Specific Assessment (SSA) application
Complete the SSA form using Ethical Review Manager (ERM). To create the SSA form:
- Login to ERM
- Select the relevant study from your list of projects
- Select the tile Create Sub-form from the list of Actions on the left-hand side
- On the Create Sub-form page, select Queensland Health as your jurisdiction and SSA Form QLD as the Main Form type, then select Create
- Complete the SSA and attach the relevant supporting documents (see further details below)
- Submit the SSA to the WMH Research Ethics and Governance Office
Supporting documentation to be attached to the SSA
The following must be included for all applications:
- Site Budget: A budget of research costs associated, both actual and in-kind, must be completed for all studies and attached to the SSA. The WMH Research Ethics Governance Officer (REGO) will obtain relevant Finance Business Partner and Director of Financial Performance for you.
- Head of Department Approval: Support of the Head of Department (HoD) must be obtained for the lead and supporting departments by having the HoD/s sign the SSA form, or attaching a letter/s of support.
The following documents should be included where required:
- HREC approval/s where WMH HREC is not the approving HREC: Please attached all HREC approval letters (both initial and any amendment approvals) where WMH is not the approving HREC.
- Contract: If you are not sure whether a contract is required, or to obtain a copy of the relevant contract template, please contact the WMH REGOs.
- Public Health Act (PHA) Approval: Please see information on use of confidential below.
- Good Clinical Practice (GCP) Certificates: Evidence of training in Good Clinical Practice (GCP) is required for investigators and study coordinators / study nurses conducting clinical trials at West Moreton Health.
GCP certification should be renewed every three (3) years and the updated certificate provided to the WMH Research Ethics and Governance Office.
If you do not have access to a paid GCP course, free online courses are available through Genesis Research Services Australia or Global Health Training Centre. - Queensland Civil and Administrative Tribunal (QCAT) Approval: QCAT approval may be required where participants with impaired decision-making capacity (i.e. unable to provide consent). For information on the QCAT approval, including access to application forms, please visit the QCAT website.
For assistance in determining whether QCAT approval is required, please contact the WMH REGOs. - CTN/CTA scheme notification: Evidence of Clinical Trial Notification (CTN) or Clinical Trial Approval (CTA) scheme is required where ‘unapproved’ therapeutic good for use in a clinical trial.
For information on the CTN and CTA schemes, please visit the Therapeutic Goods Administration (TGA) website. - Insurance and Indemnity: Where a research study is sponsored by an institution external to Queensland Health, please attach a copy of their current insurance certificate (also know as a certificate of currency).
If the sponsor is indemnifying WMH, please provide the indemnity form.
For clinical trials of medicines, the Medicines Australia Form of Indemnity for Clinic Trials – Standard is required.
For clinical trails of devices, the Medical Technology Association of Australia (MTAA) Standard Indemnity Form for a clinical investigation is required.
WMH Research Ethics Governance Officers
Email: WM_EthicsandGov@health.qld.gov.au
Where a waiver of consent has been granted by the approving HREC for the use of data from a Queensland Health facility, a legal permission to use the health information must be established.
Hospital and Health Boards Act 2011 (Qld)
Provision of confidential information may be lawful under the Hospital and Health Boards Act 2011 (Qld) (the HHB Act) where:
- every member of the research team who will be given the information is a ‘designated person’ under s139A of the HHB Act; and
- the information will be used for research for ‘evaluating, managing, monitoring or planning a health service’ under s150(a) of the HHB Act; and
- the subject matter of the research directly relates to a current ‘health service’ as defined in that Act.
Please contact the WMH REGOs for advice on whether this may be the appropriate legislation to apply to your study.
Public Health Act 2005 (Qld)
A Public Health Act (PHA) application is required when a researcher seeks to be given Information held by Queensland Health where both of the following apply:
- valid consent by, or, where relevant, on behalf of the individual about whom the Information relates has not been obtained for the disclosure and/or use of the Information for the purposes of approved research
- the disclosure is not between ‘designated persons’ as defined in s139A HHB Act for use in research ‘for evaluating, managing, monitoring or planning health services’ under s150(a) HHB Act
A data custodian may also request a PHA grant be obtained prior to disclosing the Information to provide assurance of valid authorisation to disclose.
Note that an 'opt-out' consent does not constitute a valid consent and a PHA approval may be required.
For further information on the PHA application process, including access to the application form, please visit the Health Innovation, Investment and Research Office – Use of Confidential Health Information website.
Data custodians within Queensland Health
The relevant Queensland Health Data Custodian/s is required to sign the PHA application prior to submission.